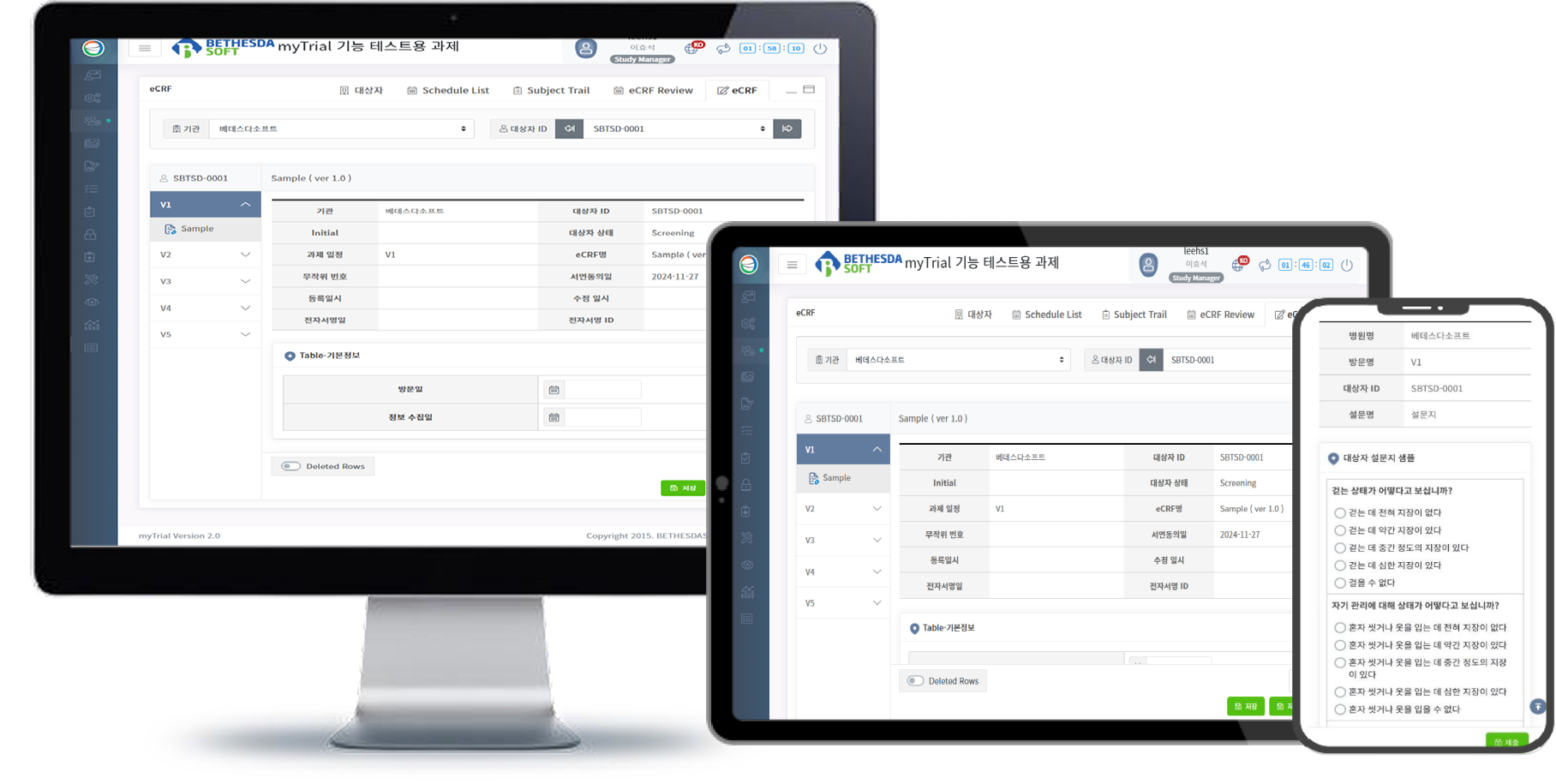
EDC / eCRF System
Introduction to e-CRF
The Korea National Enterprise for Clinical Trials (KoNECT) provides an electronic Case Report Form (eCRF) system to promote public-interest clinical research.
About MyTrial®
MyTrial® (Version 2.0) is a CDISC ODM–compliant eCRF platform developed based on KoNECT’s extensive experience supporting a wide range of clinical studies. It offers a user-friendly interface, flexible study setup, and a comprehensive suite of data management tools.
MyTrial Key Features
Easy eCRF setup and Auto Query configuration
Automated queries and conditional data search
Subject-specific notes and comment tracking
Researchers interested in using MyTrial® are requested to contact the information below. (contact: jh.jeong@konect.or.kr)